Hydrothermal polymetallic sulfides
For thirty years, we have been exploring the ocean floor and discovered nearly 150 hydrothermal sites. It was revealed that the ocean may be concealing veritable mines, when, on some sites, the presence of rich metallic deposits elements was discovered. The phenomenon of hydrothermal mineralization was revealed. During hydrothermal circulation concentrated metals are extracted, transported and deposited as metal sulphide, molecular associations of metals with sulfur, that smokers overflow with. This was the discovery of hydrothermal polymetallic sulphides.
Active smokers in the Lau Basin (Tonga, South West Pacific)
The chimneys on the left are composed mainly of zinc sulfide, copper and highly concentrated in gold.
Exploring ore sources: It begins in the depths of the Earth: It begins in the depths of the Earth ...
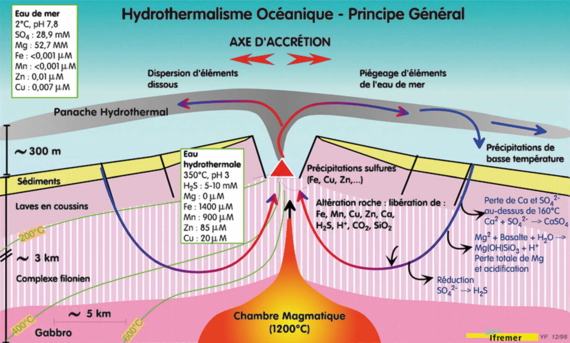
Far below the Earth's crust, a few kilometers deep, the magma chamber contains molten lava at 1200 ° C. Sea water, cold and poor in metals, but rich in salts, penetrates along faults and fissures and warms rapidly on approaching the magma chamber. Once the temperature exceeds 160 ° C, calcium sulfate CaSO4 precipitates as anhydrite, while the sulphate dissolved SO42-is gradually reduced to hydrogen sulphide H2S which remains dissolved in water.
Meanwhile, intense chemical reactions significantly affect the rocks intersected and result in total loss of magnesium, which enters the minerals resulting from altered lava. A consequence of these reactions is the acidification of fluid, increasing its ability to solubilize metals from the rocks. The high salinity of sea water also facilitates the transport of metals in the form of chloride salts. It generates hot (350 ° C) "reduced" acidic fluids, without magnesium and other metals.
Schematic cross section of rapid rift : volcanic and hydrothermal phenomena occur in the axial graben, location of separation of tectonic plates.
Hydrothermal fluids in contact with the deep rocks, are enriched in metals and undergo convection.
Of low density, these fluids rise and undergo convection, emerging as hot springs hot forming smokers in the most recent areas of fissuring in the rift. In contact with surrounding seawater (2 ° C), hydrothermal fluids cool quickly and the solid metal sulfides crystallize on the seafloor as large and fascinating chimneys.
Metallogeny
A group of ten black smokers of 2 cm in diameter, emitting a fluid containing 100 ppm of metals at 2 m / s, produces 250 tons of metal sulfides per year. An active field may consolidate some fifty of these smokers and the life span of the field can be several tens of thousands of years.
In such a system, about 1.5 million tons of sulphide (mainly iron sulfides, FeS 2) can be produced every hundred years. However it is estimated that over 95% of metals are dispersed in sea water. An efficient trap and specific geological configurations are necessary to retain a larger percentage of metals.
Hydrothermal events (chimneys and smokers) in the Lau Basin (Tonga Island, Southwest Pacific).
When the systems are stable, mounts of sulphides up as 70 meters high and several hundred meters in diameter are formed. These masses of sulfides may total tens of millions of tons. The volumes, tonnages and concentrations of elements of such recoverable deposits are identical to those of many mines operating ashore. The mounts are formed at the surface of the debris from broken chimneys cemented by subsequent flows. They are framed by a complex of active chimneys on a surface of a few tens of square meters. In contrast, on some sites, deposits are organized in and around the depressions forming the crater of the volcano. The mounts are formed not only by accumulation of chimneys broken at the surface, but also by precipitation of minerals inside, as well as replacement of bedrock. The end result is an accumulation of massive sulphides.
Thus, hydrothermal activity is a major mechanism of concentration of metals, which accumulate in the form of clusters of polymetallic sulfides: a mine for man!
Location
During exploration in 1962, early hydrothermal mineralizations associated with hot brines (70 ° C), at the axis of the Red Sea were observed. It was followed by the discovery in 1978 of the first black smoker (350 ° C) on the East Pacific Rise, about 3000 meters deep, with accumulations of sulphide deposits.
These sulphides, observed for the first time, accounted for only a few tens of thousands of tons. But as we know that the deeper fluids are the most suitable for carrying metals (high pressure so high boiling temperatures), it is certainly interesting to dive much lower. Thus, we have recently discovered the richest deposits, by diving down to 4000 meters deep. We now know several hydrothermal fields, of which the dimensions and grades of mineralization are similar to those on land mines, that is to say, several million tons.
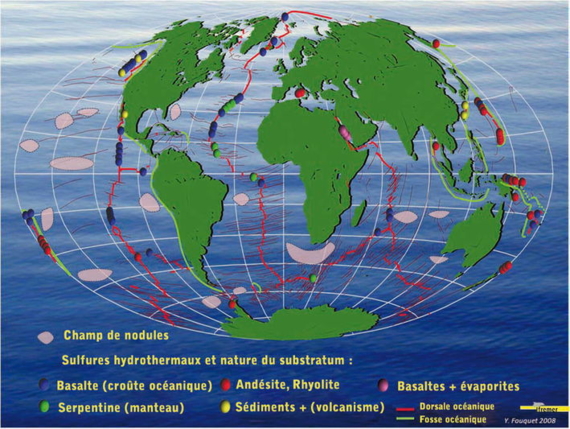
Areas of polymetallic nodules and main hydrothermal fields.
To discover polymetallic sulphide deposits, explorers have been travelling the submarine volcanic landscape in many diverse environments: fast spreading ridges, slow spreading ridges, back-arc basins and intraplate volcanoes. In the world of the deep ocean, the risks of surprise are almost infinite.
Hydrothermal sulfides proved to be very rich ores, even more so than nodules and cobalt crusts: all copper + zinc reached 20% in back-arc basins and 15% on slow spreading ridges (Atlantic) and fast spreading ridges ( Eastern Pacific). Additionally, most sites are highly rich in silver and in often gold. Some specific sites of the Atlantic, associated with mantle rocks, are also very rich in cobalt.
We now know that the sulphides have all been extracted and transported by hydrothermal fluids, i.e. by a single process. However, they present very different compositions. In fact, the factors determining their nature - substrate and metals - are found in the geodynamic environment, the nature of the bedrock affected by hydrothermal circulation, depth of water, the phenomena of phase separation or the maturity of deposits ... so many different settings, and compositions for our ores.