Polymetallic sulphides
What is a polymetallic sulphide deposit?
Behind this slightly complicated term hides a simple accumulation or "heap" of minerals composed of sulphur and metals (e.g. iron, zinc, copper...) called sulphides. We use the term "polymetallic" as there are several metals and the heaps sometimes exceed 100 m in diameter and several tens of metres in height.
Where do they form?
Polymetallic sulphide deposits are present both on the seabed and on continents. In the oceans, they start to form from depths of 800 m, above the oceanic crust (mainly composed of basalts). At lesser depths, pressure is insufficient for sulphides to form and they remain trapped in the ocean crust.
The deposits found on continents are in fact deposits formed on the seabed but which have shifted along the conveyor belt that is the ocean crust (see tectonic plates). Sometimes, this "belt" jumps and instead of passing under the continents, passes over them, depositing the sulphides (this is called obduction).
How do they form?
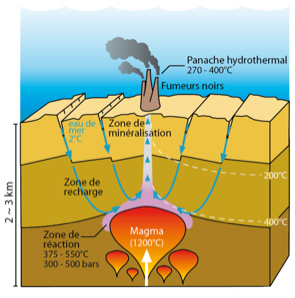
To form a good polymetallic sulphide deposit, several ingredients are necessary:
- Water
- Channels for seawater circulation : preferably fractures in the ocean crust
- Heat to get things going : ideally very hot lava located several kilometres under the seafloor (magma from the magma chamber)
Method :
Run cold seawater through the fractures of the ocean crust towards the magma chamber.
Heat the seawater by getting as near as possible to the magma. Hot water is more efficiently reactive with rock than cold water. Once heated, it becomes acidic and recovers metals (Fe, Zn, Cu, ...) and sulphur from the basalts. These elements are indispensable in the formation of sulphides. The seawater has now become hydrothermal fluid.
Continue to heat the water by approaching the magma chamber. When the hydrothermal fluid is sufficiently hot (above 500°C), its very low density will force it to work its way up to the surface. Don't forget to add the fractures, otherwise it will be trapped and unable to resurface!
Leave the hydrothermal fluid to react with the surface seawater. The mixture of acid, hot sulphur/metal laden seawater with neutral cold seawater provokes the formation of sulphides. The first sulphides will construct the chimneys (or black smokers) which will continue to grow as long as seawater mixes with hydrothermal fluid.
After a while, the chimneys break and fall and are replaced by new ones which will also collapse to gradually form the sulphide deposits.
However, between 10-40 000 years are necessary to form a deposit of 100 m in diameter by 50 m high. Many of the sulphides are "lost" during the operation and are dispersed in the ocean through hydrothermal plumes.